What is sodium carbonate?
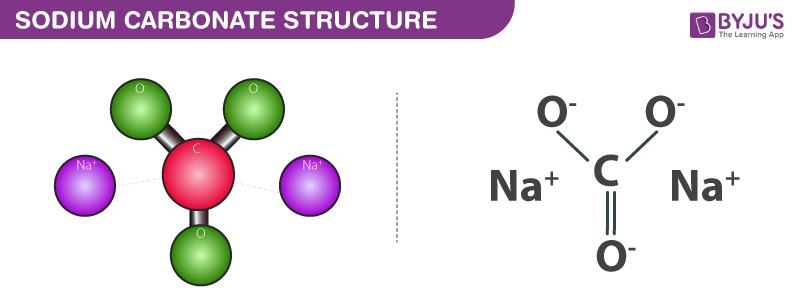
Sodium carbonate is an inorganic compound with a chemical formula Na2CO3. It is also known as Soda ash. Historically it is said to be extracted from the ashes of plants which grow in sodium-rich soils.
Physical and chemical properties of sodium carbonate
It is a white solid with no odor. The density of soda ash is 2.54 g/cm3, and the refractive index is approximately 1.535. It decomposes on heating by carbon dioxide loss and has a melting point of 851 °C.
Production of sodium carbonate
There are three ways of manufacturing sodium carbonate. Below we have discussed all the three different ways of production of sodium carbonate.
Leblanc process
Nicolas Lablanc, who was a French chemist, patented a process for producing sodium carbonate from sulphuric acid, coal, salt, and limestone in the year 1792.
Step 1: Mannheim process
Sodium chloride is treated with H2SO4 (sulfuric acid). From this reaction, we obtain sodium sulfate and hydrogen chloride. The reaction is as follows:
2NaCl + H2SO4 → Na2SO4 + 2HCl
Step 2: The salt cake and crushed calcium carbonate are reduced by heating with the help of coal. This occurs in two stages.
First stage: Carbothermic reaction – sulfate reduced to sulfide with the help of carbon and coal.
Na2SO4 + 2C → Na2S + 2CO2
Second stage: Production of sodium carbonate and calcium sulfide. The reaction is as follows:
Na2S + CaCO3 → Na2CO3 + CaS
The mixture obtained is called black ash. Soda ash is extracted from the black ash and water. Evaporation of this extract yields solid sodium carbonate. The entire process of extraction is called lixiviation.
Solvay process:
In the year 1861, Ernest Solvay, who was a Belgian industrial chemist developed a method to convert NaCl (sodium chloride) to Na₂CO₃ (sodium carbonate) using carbon dioxide and ammonia.
NaCl + NH3 + CO2 + H2O → NaHCO3 + NH4Cl
Conversion of sodium bicarbonate to sodium carbonate. The reaction is as follows:
2NaHCO3 → Na2CO3 + H2O + CO2
Regeneration of ammonia from ammonium chloride byproduct:
2NH4Cl + CaO → 2NH3 + CaCl2 + H2O
The Solvay process consumes only limestone and brine with calcium chloride being its waste product.
Hou’s process:
In the year 1930, Hou Debang a Chinese chemist developed this process. The reactions are as follows:
CH4 + 2H2O → CO2 + 4H2
3H2 + N2 → 2NH3
NH3+ CO2 + H2O → NH4HCO3
NH4HCO3 + NaCl → NH4Cl + NaHCO3
Collect the sodium bicarbonate as a precipitate and heat it up to approximately 80 °C to 95 °C to yield pure Na₂CO₃ (sodium carbonate) similar to the last step of the Solvay process.

Some Uses of Sodium Carbonate
In inclusion to its use in cleaning products, sodium carbonate is used in:
Chemical manufacturing
Therapeutic treatments
Veterinary medicine treatments
Food
Glass manufacturing
Pulp and paper products
Personal care products
Swimming pool maintenance
Cleaning Uses of Sodium Carbonate
Sodium carbonate is utilized in a few cleaning items, including green cleaning ones, because of its disinfectant properties and capacity to slice through oil and mellow water. You can discover it in clothing cleansers, programmed dishwashing cleansers, generally useful cleaners, glass cleaners, recolor removers, ledge cleaners, purifying showers, and dye.
To clean and sterilize with sodium carbonate, the Environmental Protection Agency (EPA) suggests utilizing 2 ounces for each gallon of water. This arrangement can be utilized to clean hard, non-permeable surfaces, for example, floors, dividers, baths, tile, and grout.
Sodium carbonate is viewed as an aggravation at fixations beneath 15 percent and acidic over 15 percent as indicated by the EPA, so remember this when blending your cleaning arrangements with it. Wear cleaning gloves and abstain from getting it in your eyes or mouth.
To get content on the various chemical reactions of sodium carbonate or any other chemistry-related topic such as calcium carbonate register to BYJU’S – The Learning App.









