The VSEPR Theory (Valence Shell Electron Pair Repulsion Theory) is based on the notion that all atoms have a repulsion between their pairs of valence electrons, and the atoms will constantly strive to organise themselves in a way that minimises this repulsion. The general shape of the molecule, as well as bond lengths, bond angles, torsional angles, and any other geometrical characteristics that govern the position of each atom, are all provided by molecular geometry.
The three-dimensional shape or arrangement of atoms in a molecule is known as molecular geometry or molecular structure. The atoms and molecules together constitute a compound. The shapes of molecules are determined by molecular geometry. A molecule’s shape plays a role in determining its qualities. In this article, we will discuss molecular geometry and its types.
Molecular Geometry Determination
Various spectroscopic and diffraction approaches can be used to determine molecular geometry. The specifics of the vibrational and rotational absorbance detected by IR, microwave, and Raman spectroscopy can provide information on the molecular shape. The distance between nuclei and the concentration of electron density may be used to determine the molecular structure of crystalline materials using X-ray crystallography, neutron diffraction, and electron diffraction.
VSEPR Theory
– Several methods have been devised for determining the distance between distinct atoms in a molecule, as well as the angle of the bond involved. However, using the valence shell electron pair repulsion theory, the geometries and observable forms of covalent molecules may be predicted mathematically.
– The number of valence shell electron pairs (bonded or non-bound) around the core atom determines the structure of the molecule.
– Since their electron clouds are negatively charged, pairs of electrons in the valence shell repel one another.
– The electron pairs are arranged in space around the core atom to decrease repulsion and hence increase the distance between them.
– The magnitudes of the various forms of electrical repulsions are listed in the following order:
Lone pair – Lone pair > Lone pair – Bonding pair > Bonding pair – Bonding pair
- The bond angles of the molecule or ion are changed by these repulsive forces.
- When two pairs of electrons are as far apart as feasible, the electrical repulsion between them is minimal.
How Can VSEPR Be Used to Predict Molecular Geometry?
The following processes must be performed in order to identify the shape of a molecule:
1. Since it has the greatest ability to share electrons with other atoms in the molecule, the least electronegative atom must be designated the centre atom.
2. Then add up the total amount of electrons in the core atom’s outermost shell.
3. Count the total number of electrons the other atoms have and utilise them to form bonds with the centre atom.
To get the valence shell electron pair number, or VSEP number, add these two numbers together.
Types of Molecular Geometry
Linear molecular geometry
Two molecules are connected to the centre atom in the linear molecular geometry. To decrease their repulsion, they positioned themselves in the opposite way. This structure has an 1800 bond angle. Example: BeCl2
Tetrahedral molecular geometry
A central atom is positioned in the core of a tetrahedral molecular geometry, with four substituents arranged at the corners of the tetrahedron. The 109028′ structure’s bond angle. Example: CH4
Trigonal bipyramidal geometry
A molecular geometry with one atom in the centre and five additional atoms at the corners of a triangular bipyramid is known as a trigonal bipyramid formation. Example: PF5. The bond angles of this geometry are 900 and 1200
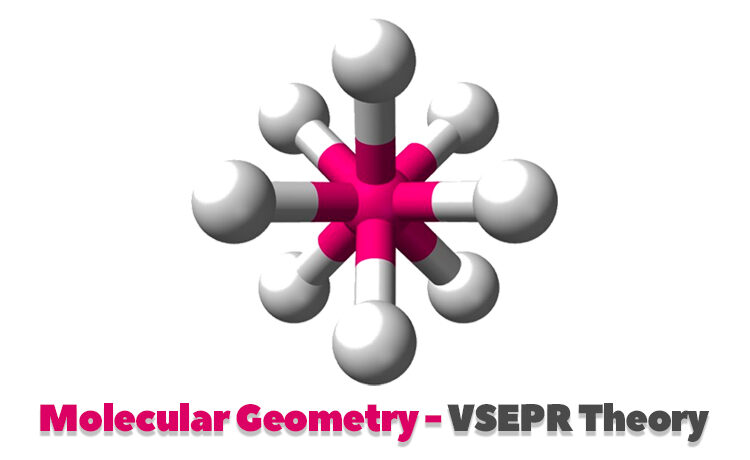
Octahedral molecular geometry
Octahedral molecular geometry describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically clustered around a central atom, defining the vertices of an octahedron. This geometry has a bond angle of 900
Trigonal planar geometry
BF3 and BCl3 are two examples of this type of geometry. A centre atom is connected to three atoms arranged in a triangle configuration around the core atom in a trigonal planar compound. The geometry has a 1200 bond angle.
Summary
The three-dimensional arrangement of the atoms that make up a molecule is known as molecular geometry. It contains data about the molecule’s overall structure, as well as bond lengths, bond angles, torsional angles, and any other geometrical characteristics that govern each atom’s location. Reactivity, colour, phase of matter, polarity, biological activity, and magnetism may all be predicted by knowing a molecule’s geometry.